Our seroconversion panels are intended for use by diagnostic manufacturers and researchers during assay development, evaluation and troubleshooting test methods. Each sample is a unique plasma sample drawn during a developing virus or infection.
COVID-19 Seroconversion Panels
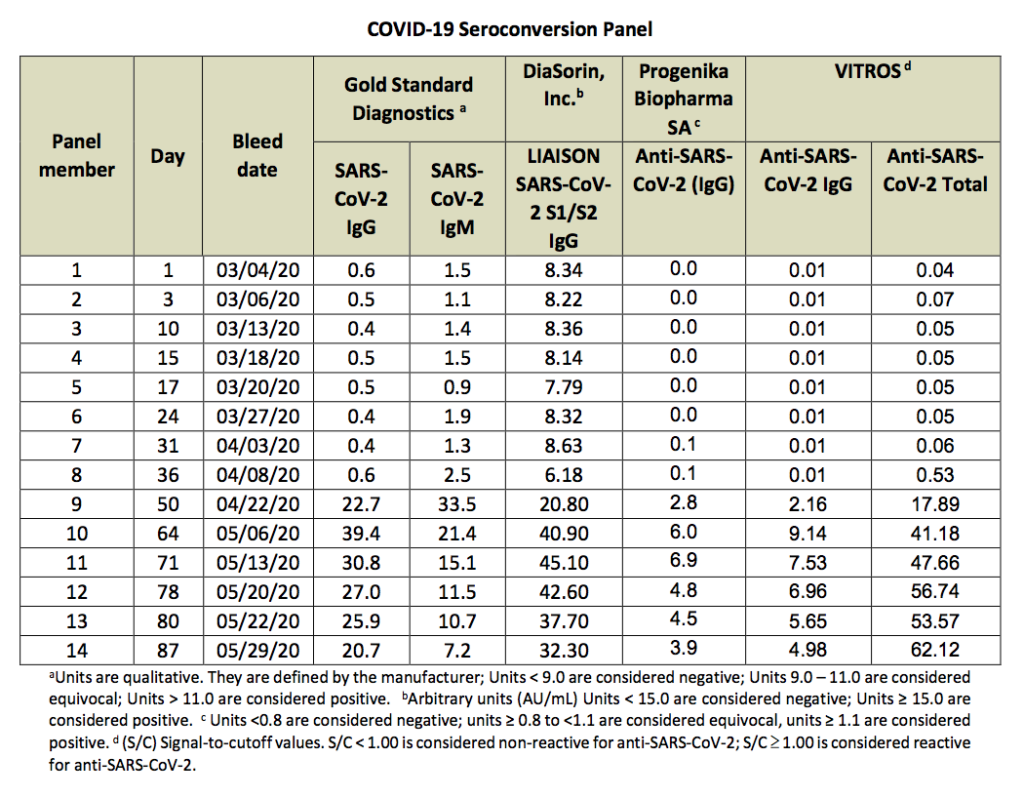
The COVID-19 Seroconversion Panel consists of 14 members with each member containing 1 vial of 1.0 mL of human plasma. Panel members collected in this longitudinal series are from a single donor during the progression of a SARS-CoV-2 infection. This panel illustrates the onset and decline of IgM, IgG and Ig total SARS-CoV-2 virus antibody titers over a period of 87 days. Panel members are undiluted, naturally occurring plasma samples collected in 4% sodium citrate. Units were aseptically filtered. No preservative has been added. All panel members are ready to use.
Commercial COVID-19 serial seroconversion panel for validation of SARS-CoV-2 antibody assays
In this study, a seroconversion panel obtained from a single patient was tested against three commercially available test kits for detecting anti-SARS-CoV-2 antibodies. This seroconversion panel is one of the first available for SARS- CoV-2. The advantage of this characterized seroconversion panel is the expansive time period (samples taken over almost 90 days) providing an extensive record of seroconversion in this single donor.
COVID-19 J&J, Moderna and Pfizer Vaccine Panels
Access Biologicals is now collecting samples from donors being vaccinated for COVID-19.
- Longitudinal Vaccine Panel
6 samples per subject
Following vaccinated donors pre-vaccine to 6 months post-vaccination series completed
Sample 1: Pre Vaccine
Sample 2: Post 1st dose
Sample 3: Post 2nd dose
Sample 4: 1.5 months post 2nd dose
Sample 5: 3.5 months post 2nd dose
Sample 6: 6.5 months post 2nd dose
• Serum only
• 15 donors
• 20 panels available
- 14 Subject Panel
3 samples per subject
Sample 1: < 1 day before their vaccination
Sample 2: 2 weeks post their vaccination
Sample 3: 2 months post their vaccination
• Some subjects with previous COVID infections
• Serum only
• 1-2 mL samples
• Methodology: DiaSorin LIAISON SARS-CoV-2 S1/S2 IgG
- 15 Subject Panel
3 samples per subject
Sample 1: ≤ 2 days before the first vaccination
Sample 2: ≤ 2 days before their second vaccination
Sample 3: 13-15 days post their second vaccination
• Some subjects with previous COVID infections
• Serum only
• 1mL samples
• Methodology: DiaSorin LIAISON SARS-CoV-2 S1/S2 IgG
- 30 Subject Panel
3 samples per subject
Sample 1: ≤ 2 days before the first vaccination
Sample 2: ≤ 2 days before their second vaccination
Sample 3: 13-15 days post their second vaccination
• Some subjects with previous COVID infections
• Serum and K2 EDTA Plasma
• 1mL samples
• Methodology: DiaSorin LIAISON SARS-CoV-2 S1/S2 IgG
Contact us for more information or to request a custom panel.
Epstein-Barr Virus (EBV) Seroconversion Panel
- EBV001SCP
The EBV Seroconversion Panel consists of 14 members with each member containing 1 vial of 1.0 mL of human plasma.
Panel members collected in this longitudinal series are from a single donor during the progression of an early EBV infection. This panel illustrates the onset and follow up of IgM and IgG Epstein-Barr virus antibodies over a period of 182 days. Panel members are undiluted, naturally occurring plasma samples collected in 4% sodium citrate. Units were aseptically filtered. No preservative has been added. All panel members are ready to use.
Full product description here.
HAV Seroconversion Panels
- HAV001SCP
This HAV Seroconversion Panel consists of 23 members with each member containing 1 vial of 1.0 mL of human plasma.
Panel members collected in this longitudinal series are from a single donor during the progression of an early HAV infection. This panel illustrates the onset and decline of IgM and Ig total Hepatitis A virus antibodies and RNA titer over 120 days. Panel members are undiluted, naturally occurring plasma samples collected in 4% sodium citrate. Units were aseptically filtered. No preservative has been added. All panel members are ready to use.
Full product description here.
- HAV002SCP
This HAV Seroconversion Panel consists of 13 members with each member containing 1 vial of 1.0 mL of human plasma.
Panel members collected in this longitudinal series are from a single donor during the progression of an early HAV infection. This panel illustrates the onset and decline of IgM and Ig total Hepatitis A virus antibodies and RNA titer over 139 days. Panel members are undiluted, naturally occurring plasma samples collected in 4% sodium citrate. Units were aseptically filtered. No preservative has been added. All panel members are ready to use.
Full product description here.
- HAV003SCP
This HAV Seroconversion Panel consists of 13 members with each member containing 1 vial of 1.0 mL of human plasma.
Panel members collected in this longitudinal series are from a single donor during the progression of an early HAV infection. This panel illustrates the onset and decline of IgM and Ig total Hepatitis A virus antibodies and RNA titer over a period of 108 days. Panel members are undiluted, naturally occurring plasma samples collected in 4% sodium citrate. Units were aseptically filtered. No preservative have been added. All panel members are ready to use.
Full product description here.
Human Parvovirus B19 (B19V) Seroconversion Panel
- B19V001SCP
The B19V Seroconversion Panel consists of 6 members with each member containing 1 vial of 1.0 mL of human plasma.
Panel members collected in this longitudinal series are from a single donor during the progression of an early parvovirus B19 infection. This panel illustrates the profile of IgM and IgG parvovirus B19 antibodies and DNA titer over 19 days. Panel members are undiluted, naturally occurring plasma samples collected in 4% sodium citrate. Units were aseptically filtered. No preservative has been added. All panel members are ready to use.
Full product description here.
If you have specific panel needs please contact us at info@accessbiologicals.com or click here to send us a contact form.
Need Help? Contact Us

